Allylic carbocations carbocation with a vinyl group as a substituent next to a double bond cc c 221 allyl carbocations are stabilized by resonance cc c cc c c c c cc c cc c recall from chapter 1 8.
Vinyl carbocation hybridization.
The vinyl carbocation a primary carbocation.
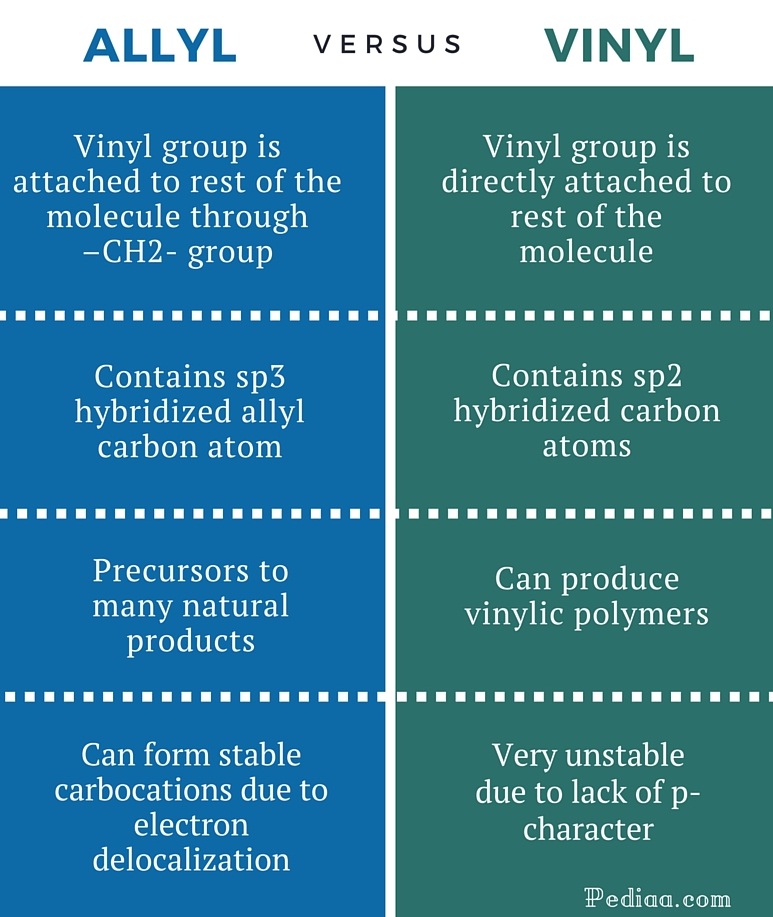
An allylic carbon is one that is directly attached to a pi bond.
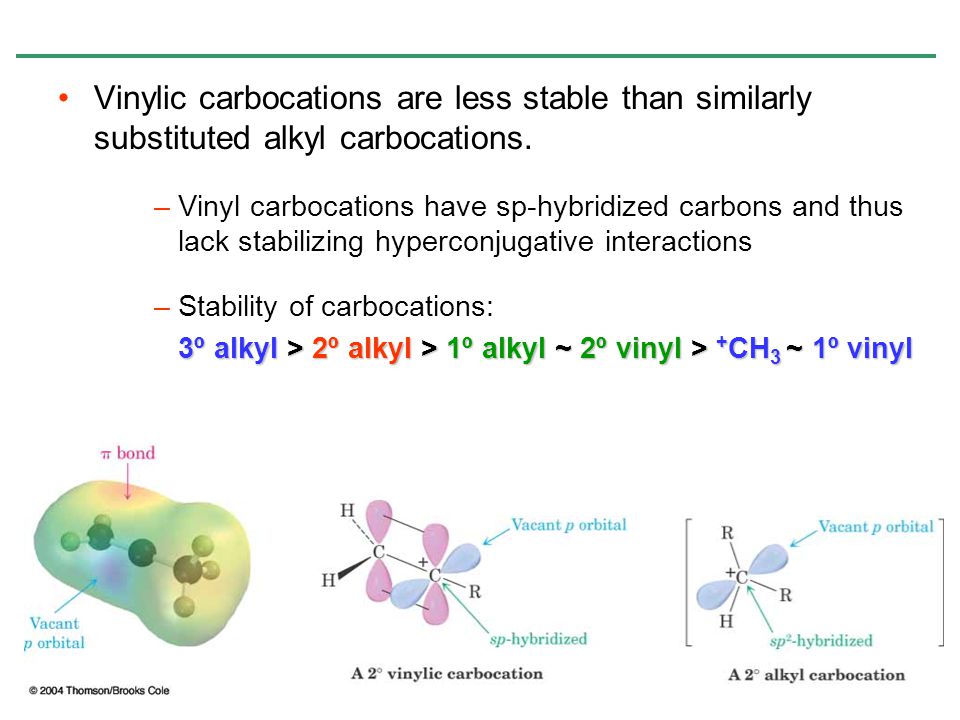
Carbon with two other atoms attached prefers sp hybridization and a linear geometry.
The relatively high energy of a carbocation however means that.
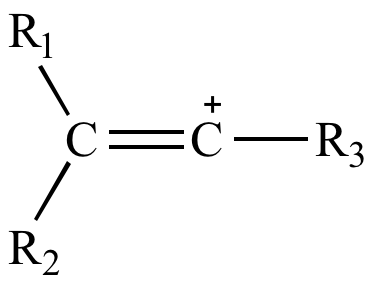
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
A vinyl cation is a positively charged molecule a cation where the positive charge is located on a vinyl group ch ch2.
A vinyl carbocation has a positive charge on the same carbon as the double bond.
An allylic system has a minimum of 3 carbons.
The n in benzonitrile can be perfectly described using a sp hybridization in all the resonance structures present.
Hydride shifts and carbocations.
Enjoy the videos and music you love upload original content and share it all with friends family and the world on youtube.
Structure of vinylic carbocation.
Allylic carbocations benzylic carbocations and aryl carbocations all can be related to vinyl carbocations.
The vinyl carbocation prefers a linear geometry which was well expected from its sp hybridization.
Vinyl carbocation is unstable.
The allylic carbon and the nearby double bond.
In the first mechanism step the alkyne is protonated by hydronium ion a strong acid to produce a resonance stabilized secondary vinylic carbocation shown in red.
Do not confuse an allylic group with a vinyl group.
Although simple vinyl cations are very unstable and are rarely formed the acylium ion produced in the friedel crafts reaction is relatively stable.
Acid catalyzed hydration of phenyl acetylene a terminal alkyne involves a vinylic carbocation intermediate.
The hybridization of a vinyl carbocation is sp hybirdized.
In the case of vinylic or arylic carbocations some of the resonance structures you draw are unreasonable if you take the proper geometry of the cation into account which won t be linear in the case of a vinylic carbocation.
Vinyl carbon allylic hydrogen sp2 hybridized vinyl hydrogen oh cl allyl alcohol allyl chloride 10 2.